A retrospective analysis of more than 1,100 acute myeloid leukaemia (AML) patients confirms that the ELN-2017 classification allows better risk assessment of AML patients receiving induction therapy compared to the ELN-2010, regardless of age.

Outcome in patients with acute myeloid leukemia ranges from death within a few days after diagnosis to cure. Knowledge of the mutation status of various genes at diagnosis has improved our ability to select initial and subsequent treatment and prediction of long-term treatment outcome. This retrospective study confirms the revised 2017 European LeukemiaNet genetic risk stratification for acute myeloid leukaemia.
Herold, T., et al. Leukemia 220;34(12):3161-72
In 2010, the European LeukemiaNet (ELN) guidelines introduced a genetic risk stratification to facilitated a better diagnosis and management of adult acute myeloid leukaemia (AML) patients.1 In 2017., these guidelines were updated to include an updated genetic risk stratification system incorporating additional cytogenetic and molecular prognostic factors (mutations in ASXL1, RUNX1 and TP53, impact of FLT3 internal tandem duplications, CEBPA).2 The updated guidelines have been widely adopted, but remain to be validated in large cohorts of AML patients.
The study at hand aimed to validate the prognostic significance of the revised ELN-2017 risk classification in 116 AML patients receiving induction chemotherapy across a broader age range (18-86 years).3 In the ELN-2017 risk stratification system, the distinction between the intermediate-I and intermediate–II groups has been eliminated,2 complicating side-by-side comparisons, after which it was decided to treat them as one group for 771 patients. For this analysis 345 patients with cytogenetically normal (CN)-AML were excluded, to avoid bias due to overrepresentation of CN-AML. It resulted in reclassification of 204 patients (26.5%) into a higher or lower prognostic category.
In this cohort 40%, and 51% of patient’s ≥60 years, were classified as adverse-risk by ELN-2017. Outcome analyses were done for all 1116 patients with a median follow-up for survivors of 98 months. Patients in the favourable, intermediate and adverse-risk categories showed progressively worse RFS and OS (Figure 1). In the 599 patients aged <60 years, estimated 5-year overall survival (OS) rates were 64% for favourable-risk, 42% for intermediate-risk and 20% for adverse-risk patients. Among the 517 patients aged ≥60 years, corresponding 5-year OS rates were 37%, 16% and 6%. As such, these findings seem to validate the prognostic significance of the revised ELN-2017 risk classification across a broad age range. Of note, the prognostic relevance of the ELN-2017 categories was less clear in very old patients (≥75 y) who still undergo intensive treatment.
Current treatment guidelines suggest an allogeneic stem cell transplantation (alloSCT) as the preferred post-remission treatment for suitable patients with an adverse genetic risk.2,4 In this study, 664 patients reached a CR after therapy of whom 109 and 46 underwent allogeneic or autologous transplantation (autoSCT) while in first CR, respectively. The remaining 509 patients received chemotherapy as post remission therapy. Only in the adverse-risk group AlloSCT was shown to be associated with an improved OS (p= 0.05). Of note, since post remission alloSCT assignment was not randomized, other factors besides genetic risk may have affected therapeutic decisions and thus biased these results.3
Outcomes of specific genetic subsets within the ELN-2017 risk categories further support the changes introduced in the ELN-2017.2 Patients with biallelic CEBPA mutations or inv(16) had particularly favourable outcomes, while patients with mutated TP53 and a complex karyotype had especially poor prognosis. In addition, DNMT3A mutations were associated with an inferior OS in all ELN-2017 risk groups.3 However, predictions of long-term treatment outcomes based on pre-treatment genetic characterization alone are far from perfect. Age, comorbidities, performance status and other important risk factors are not reflected in the ELN categories.2 Moreover, analyses of measurable residual disease (MRD) during and after treatment by flow cytometry, quantitative PCR or next- generation sequencing have emerged as novel tools to assess response to therapy and prognosis as well.5,6 This would suggest the development of algorithms that integrate pre-treatment risk factors and longitudinal MRD measurements to guide individualized AML treatment would be beneficial.
In summary, this study confirms that the ELN-2017 classification allows better risk assessment of AML patients receiving induction therapy compared to the ELN-2010, regardless of age.3 It is important to keep in mind that risk classification systems must always be interpreted in conjunction with specific patient characteristics and treatment regimens, which may change over time.
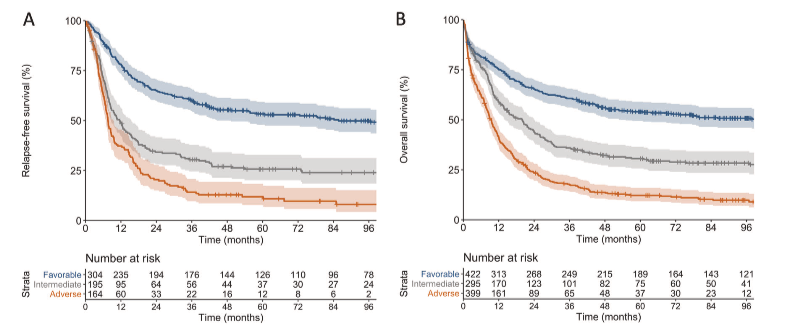
Figure: Outcomes of patients according to the ELN-2017 genetic risk groups. A) Relapse free survival and B) Overall survival. 3
References